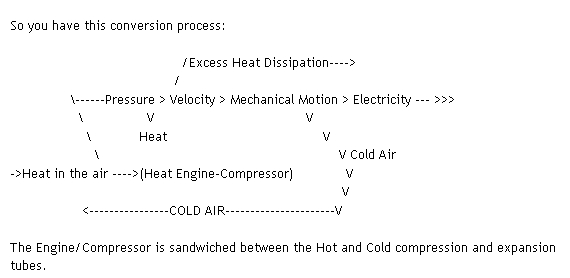
Bumpkin wrote: I don't know if I understand you to mean that you could collect energy by simply exhausting the cold gas - the temp drop doesn't represent power until it collects ambient heat, a process that would in that case happen externally and be totally lost.
My understanding of gas under pressure is that the pressure (PSI) would be more or less equally distributed along the walls of the long tube that the "compressor" is pumping air into regardless of temperature changes along the way.
The air, however, is being continually cooled as it travels down the tube. Therefore, contracting and loosing pressure along the way. What you would have exiting the tube into the turbine would be very dense cool air but presumably the very hot air behind it would tend towards lending pressure to it as the pressure should be equal at any point.
My understanding; due to studying the principles involved in gas liquefaction which uses a similar process of compression and pre-cooling of a gas and injection of that pre-cooled condensed gas into a turbine; is that the condensed gas is in a sense "wound up" or actually "compressed" not by pressure so much as by cooling, like a compressed spring and when released into the turbine through a nozzle the cool compressed air expands rather explosively, like a spring suddenly released, and so powers the turbine.
The gas or compressed air, once released, requires HEAT in order to expand.
If the turbine is well insulated, NO EXTERNAL HEAT IS AVAILABLE for it to draw upon for energy to do the expanding. Regardless, the gas expands rapidly anyway and does power the turbine by such expansion drawing upon its own "INTERNAL LATENT HEAT" to do so.
I am not a Physicist and so can not say exactly how or why this happens, but the result is that the gas, having thus expanded, in spite of no external heat being available to do the expanding,
drops in temperature precipitously due to being forced to draw upon it's own INTERNAL HEAT RESERVE. Remember that the gases in the air contain a great deal of latent heat well below ambient temperatures.
There is no "Ambient Heat" involved at this point. None is available. More than likely the COOL pressurized air, as it is released into the turbine is alredy BELOW AMBIENT. So where does the power to turn the turbine come from? From the latent
internal molecular heat within the gas or air itself. This is somewhat difficult to conceptualize but it is a process in common use today, even now as we speak. It is used in various cooling processes, air-cycle refrigeration, gas liquefaction, cryogenics, refining processes, etc. etc.
One of the reasons it (the "expansion turbine") is so widely used is that much of the energy to compress the air is recovered when it is then re-expanded through the turbine. Like compressing a spring and letting it go again.
But under ordinary circumstances it takes more energy to compress the air than you get back when it expands. Conventional compressors generally eat and waste a lot of power. Theoretically though, a compressor that runs on ambient heat would have a virtually inexhaustible reserve of energy to draw upon, so if you only get 10% back at the turbine that is 10% of freely available, inexhaustible ambient/solar heat/energy.