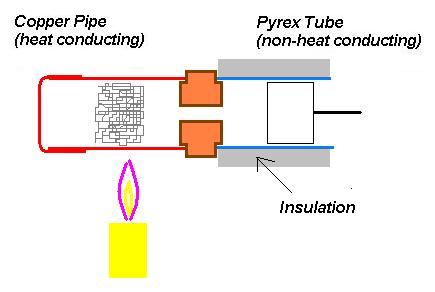
I have often argued this point, especially with those well educated in thermodynamics and the "Carnot cycle" as it contradicts what are considered well established principles:derwood wrote:...One thing I have noticed is that with all of my attempts the engines did show signs of running but only after the entire engine got extremely hot (piston cylinder too). I have been using all steel construction making sure to use very thin steel for the heat chamber. I now realize that material selction is very important. pyrex tube or ss is for the heat chamber is recomended because it does not conduct heat very good. Many people say this is true because heat is not conducted lengthwise to the piston cylinder. If this was true then thin mild steel should also work. It takes quite a while for the heat to conduct to the piston cylinder when using thin mild steel but it just does not want to work. many people report that when using pyrex and ss the piston cylinder heats up very quickly but with steel it takes a very long time. I think that pyrex and ss work good because like all materials that conduct heat poorly, they are a very good insulator as well. Even though the thin steel does not cunduct heat down it's length very well it is not a good insulator. It could be that the air (shock wave) inside the engine needs stay very hot, right up until the point of entering the piston cylinder. The thin steel does not allow this, It cools the air prematurely. The temperature difference is very low by the time it reaches the cylinder. You might say it diffuses the shock wave. With the pyrex and ss the hot air is insulated and maintains a much higher temperature. The temp difference will be higher. This might explain why the piston cylinder heats up quicker with the pyrex. This also may explain why one of my engines actually ran. It was the only one that used a cast iron cylinder, perhaps this cylinder cooled the air a little better than the steel cylinders, just enough to keep a higher temp difference but barely enough to run. Although these conclusions may not be correct, my many hours of reading and testing do give them support.
It is generally believed that for a heat engine to work there has to be a "heat sink". That is, one end of the engine has to be cold so that the heat can be transferred.
Personally, from my own observations, I think that this is wrong, or at least not necessarily applicable in the case of the "Lamina Flow" Stirling.
When a gas is heated and expands and does "work" pushing a piston it uses up energy. As a result of this expenditure of energy it uses up HEAT. That is, the heat is converted into the kinetic energy/momentum of the piston/flywheel, along with friction and so forth.
A Lamina Flow Stirling "Cycle" bears no resemblance to the "Carnot" cycle. I think what happens in the Lamina Flow Stirling is that the heat is almost entirely, if not entirely CONVERTED so that as a result, no "heat sink" is necessary.
In observing many Lamina Flow Stirling Engines, it appears to me that the engines are generally running at a rate of speed which precludes heat transfer to the sink. In other words, the engine is running too fast for heat to be dissipated through the glass or Pyrex test tube which conducts heat very slowly. It seems to me, therefore, that the only logical explanation that remains for why the piston is able to return at all is that the air has given up its energy to the piston and as a result it gets cold and contracts drawing the piston back inwards with it. I think this is especially true or evident with a "free piston" type engine as there is no flywheel to push the piston back inward. If things are moving too fast for heat transfer to the "sink" to take place then as far as I can deduce, the only thing that could be drawing the piston back is the cooling and contraction of the gas or air due to giving up energy to the piston. The heat is converted rather than transferred. I've been told many times that this is "impossible" and that it would be a violation of the second law of thermodynamics and the "Carnot Cycle" which REQUIRE that at least SOME heat be transferred. You can't convert ALL of the heat. But IMO the Lamina Flow Stirling seems to prove differently. That is, ALL the heat that goes to heat and expand the gas is converted so that no heat sink is required. In-fact, the presence of a heat sink could only diminish the performance of the engine as it is WASTING heat by letting it out at the sink so less heat is CONVERTED.
My conclusion then would be that as far as possible, the piston cylinder should be entirely NON-HEAT CONDUCTING. In that way ALL the heat is used to push the piston and none is lost to the cylinder walls robbing power. This contradicts very firmly established "Laws" of thermodynamics, nevertheless, that is what I see.