My question is, does the Ambient heat running a heat engine actually get converted as Tesla imagined or is the heat just intercepted in its passage but continues on its way like the water over a water wheel?
I thought up an experiment which could demonstrate the theory one way of the other.
Take two identical LTD type Stirling Engines and set them on top of two identical insulated Styrofoam or perhaps wooden pans full of equal amounts of ice. (Something non-heat conducting at any rate) so that the Ambient heat can only reach the ice by passing through the engines (predominantly anyway). Have a small hole in the bottom of each pan with a water glass underneath.
Now set the engine on the left running but leave the other engine idle or disabled.
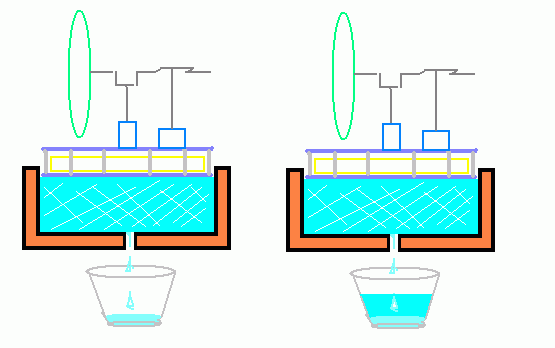
If Tesla was right, the ice in the pan on the left with the running engine on top should melt more SLOWLY than the pan on the right with a non-operational engine since the running engine is converting some portion of the heat trying to reach the ice into another form of energy.
If this does not happen, then I would have to conclude that there is something wrong with Tesla's theory and possibly something wrong with our general understanding of how a heat engine operates.
At this point I do not have a couple of identical engines to run this experiment immediately but I thought someone else here might.
Tesla wrote:
If Tesla's assumptions were correct, then in this experiment, the ice in the pan with the engine RUNNING on top should melt more slowly than the one with an engine not running. The running engine, to one degree or another, according to its efficiency, acting somewhat like a refrigeration unit keeping the ice cold or converting a portion of the heat into mechanical motion."...Heat, though following certain general laws of mechanics, like a fluid, is not such; it is energy which may be converted into other forms of energy as it passes from a high to a low level.... If the process of heat transformation were absolutely perfect, no heat at all would arrive at the low level, since all of it would be converted into other forms of energy.... We would thus produce, by expending initially a certain amount of work to create a sink for the heat to flow in, a condition enabling us to get any amount of energy without further effort. This would be an ideal way of obtaining motive power.
"We do not know of any such absolutely perfect process of heat-conversion, and consequently some heat will generally reach the low level, ...But evidently there will be less to pump out than flows in, or, in other words, less energy will be needed to maintain the initial condition than is developed by the fall, and this is to say that some energy will be gained from the medium. What is not converted in flowing down can just be raised up with its own energy, and what is converted is clear gain. Thus the virtue of the principle I have discovered resides wholly in the conversion of the energy on the downward flow."
At lest there should be some measurable difference in the amount of melt-water accumulated in each water glass regardless of the efficiency of the engines used.
I have pondered this question for some time. Is the heat passing through a heat engine actually converted or is it merely intercepted? Energy can be derived from water passing over a water wheel, but the water itself is in no way changed or converted into something else.